Candle Flame

Question - Please explain the anatomy of a candle flame. How does it burn? Why it is not a reversible reaction? What conditions will affect the burning of the candle and in what regions?
----------------------------------------
Combustion chemistry is very complicated! Many, many reactions are occurring sequentially and simultaneously. Scientists and engineers do not fully understand the chemistry and mechanics of a candle flame (or other types of flames), but quite a bit IS known.
* In general, the fate of the wax molecules is this: the heat of the candle flame first melts the wax, and it rises up the candle wick by capillary action. Farther up the wick, the greater heat vaporizes the wax molecules, which move from the wick into the surrounding space. The heat of the flame and reactive molecules (free radicals) in the flame break apart the wax molecules, in particular stripping hydrogen atoms from the carbon-chain backbone. Some of the carbon chains fragment into gaseous carbon (C2) and into small (typically two-carbon atom containing) molecules and molecular fragments. The hydrogen atoms stripped from the wax molecules eventually combine with oxygen atoms from the air to form water molecules. The carbon atoms eventually combine with oxygen to form carbon monoxide and carbon dioxide, but first many of them combine to form very large (as far as molecules are concerned) clumps of carbon-rich solid material, called soot. Some of this soot burns to make carbon dioxide in the candle flame, and sometimes some of it escapes the flame.
* Several zones of a candle flame can be seen with the eye. At the bottom is a region that gives off blue light. This light is actually molecular emission from gaseous carbon, C2. Further up the flame is a region that is substantially opaque and which gives off yellow light. This is known as the "incandescent region", and is where hot soot particles glow, giving off light like the filament of a light bulb. The inside part of the flame, near the wick, is oxygen-deficient, and most of the reactions that occur are heat-induced fragmentations and rearrangements. In the outer regions, where oxygen can enter from the surrounding air, the fragments combine with oxygen, eventually forming water and carbon dioxide.
* Many factors that affect the burning of a candle. Most of them are of the type that are difficult to vary, such as the air pressure, concentration of oxygen, thermal conductivity of air, and the buoyancy of the hot reaction products. One factor that is easy to vary, however, is wind. A good breeze, or even a person's breath, can blow the hot flame gases away from the source (the candle and wick), interrupting the process and extinguishing the flame.
Richard E. Barrans Jr., Ph.D.
Director of Academic Programs
PG Research Foundation, Darien, Illinois
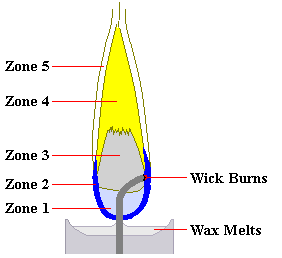
Zone 1 (Non-Luminous Zone) - Fuel on the wick evaporates. There is insufficient oxygen for fuel to burn. Temperature is about 600°C near the wick.
Zone 2 (Blue Zone) - There is a surplus of oxygen and the flame burns clean and blue. Temperature is around 800°C. In a candle the heat from this zone melts nearby wax to allow for wicking.
Zone 3 (Dark Zone) - Pyrolysis (cracking) of the fuel begins due to the shortage of oxygen creating minute carbon particles. The temperature is about 1,000°C.
Zone 4 (Luminous Zone) - This area is bright yellow. There is still insufficient oxygen for complete burning so pyrolysis continues and larger carbon particles are produced. The temperature is around 1,200°C.
Zone 5 (Veil) - There is oxygen surplus in this non-luminous zone and carbon particles burn faster and more completely at the boundary between Zone 4 and Zone 5. The temperature is around 1,400°C. If a draft lowers the temperature below 1,000°C, soot particles cease burning and end up on your pot or in your lungs.
Back to Main Chemistry page
Back to MAIN Pysanka home page.
Back to Pysanka Index.
Search my site with Google
Under Construction

Color-shift deliberately applied on the image of a burning candle in order to
enhance the visibility of the color-zones


