Wax Removal
Chemical Dissolution
Wax Removal
Chemical Dissolution

Chemical dissolution is a much more modern and less labor-intensive way of removing wax. It is quite simple: a pysanka is placed into a degreasing solution, and the wax dissolves off. What could be easier? Not much, really, but because of the fumes involved, potential toxicities, and the flammability of all the available solvents, it is not the method of choice for most people.
A popular aphorism , long known by all chemistry students, used for predicting solubility is "like dissolves like". This statement indicates that a solute will dissolve best in a solvent that has a similar chemical structure to itself. What this means, in simple terms, is that substances (solutes) will dissolve in liquids (solutions) with a similar molecular structures. The similarities, in this case, have to do with the types of chemical bonds that hold a compound together.
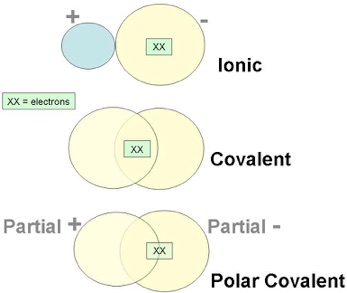
Broadly speaking, the chemical bonds can be ionic or covalent. Crystalline solids are held together by the attraction of two oppositely charged ions (ionic or polar bond). Another way of bonding, the covalent bond, occurs when the bond is formed by a shared pair of electrons (non-polar bond). There are other in-between cases where the electron sharing is not equal, called polar covalent bonds, but those are beyond the scope of this discussion.
Similarly, “like” liquids should mix together, and blend, while “unlike” liquids won’t, and will separate out. You’ve all seen what happens when oil and water are mixed– they will quickly separate into distinct layers.
Many commercial solvents are mixes of a variety of non-polar liquids, rather than being a single solvent, and most of them will dissolve beeswax and similar substances. They vary in toxicity, carcinogenicity, flammability, and density. But non-polar solvents, as can be expected by their chemical similarities, will mix together pretty well.
Alcohols (ethanol, methanol, etc.) fall somewhere towards the middle of the polar/non-polar spectrum, with characteristics of both. They will mix with both polar substances (e.g. scotch and water) and with non-polar ones (e.g. perfumes, which are mixes of essential oils and alcohol). While alcohol can be used to remove wax (non-polar), the water in it (because alcohols are not usually 100% pure) can cause dyes (polar) to run.
On the following pages you can read about various commercial solvents and their uses in pysankarstvo, solvent safety, and techniques of applied chemical dissolution for pysankarstvo.
Back to Main Wax Removal page
Back to Main Pysankarstvo page
Removing wax by DISSOLUTION
