Solvent Safety

Under construction
There are many household and professional solvents that can be used as degreasers in pysankarstvo. Unfortunately, all of the non-flammable degreasers have been shown to be deleterious to the environment and have been taken off the market. Energine (original formula), Carbosol and the like are no longer available–this is good for Mother Earth, but not so good for pysankary.
I thought it might be good to understand what the various ratings of “Flammable,” Combustible,” and “Inflammable” really mean.
The U.S. Occupational Health and Safety Administration (OSHA) defines a combustible liquid as "any liquid having a flash point at or above 100 deg. F (37.8 deg. C), but below 200 deg. F (93.3 deg. C). Compare this definition to flammable, which indicates a liquid that is even easier to ignite (flash point below 100 oF). Spontaneously combustible materials can undergo combustion and burn without the addition of heat or flame; arguably, the term "spontaneously flammable" is more appropriate.
Proper storage and use of combustible materials is absolutely critical in maintaining a safe work place. Avoid placing or using combustible materials near sources of heat or flame (direct sunlight, furnaces, pilot lights etc.). Use caution when disposing of combustible materials such as linseed oil-soaked rags (which can spontaneously combust). When dispensing combustible or flammable liquids, keep in mind that static electricity poses a very real threat; obey all standard bonding and grounding practices.
From the CCOHS and OSHA
What are flammable and combustible liquids?
Flammable and combustible liquids are liquids that can burn. They are classified, or grouped, as either flammable or combustible by their flashpoints. Generally speaking, flammable liquids will ignite (catch on fire) and burn easily at normal working temperatures. Combustible liquids have the ability to burn at temperatures that are usually above working temperatures.
There are several specific technical criteria and test methods for identifying flammable and combustible liquids. Under the Workplace Hazardous Materials Information System (WHMIS), flammable liquids have a flashpoint below 37.8°C (100°F). Combustible liquids have a flashpoint at or above 37.8°C (100°F) and below 93.3°C (200°F).
Flammable and combustible liquids are present in almost every workplace. Fuels and many common products like solvents, thinners, cleaners, adhesives, paints, waxes and polishes may be flammable or combustible liquids. Everyone who works with these liquids must be aware of their hazards and how to work safely with them.
What is a flashpoint?
The flashpoint of a liquid is the lowest temperature at which the liquid gives off enough vapour to be ignited (start burning) at the surface of the liquid. Sometimes more than one flashpoint is reported for a chemical. Since testing methods and purity of the liquid tested may vary, flashpoints are intended to be used as guides only, not as fine lines between safe and unsafe.
Does the liquid itself burn?
Flammable and combustible liquids themselves do not burn. It is the mixture of their vapours and air that burns. Gasoline, with a flashpoint of -40°C (-40°F), is a flammable liquid. Even at temperatures as low as -40°C (-40°F), it gives off enough vapour to form a burnable mixture in air. Phenol is a combustible liquid. It has a flashpoint of 79°C (175°F), so it must be heated above that temperature before it can be ignited in air.
What are flammable or explosive limits?
A material's flammable or explosive limits also relate to its fire and explosion hazards. These limits give the range between the lowest and highest concentrations of vapour in air that will burn or explode.
The lower flammable limit or lower explosive limit (LFL or LEL) of gasoline is 1.4 percent; the upper flammable limit or upper explosive limit (UFL or UEL) is 7.6 percent. This means that gasoline can be ignited when it is in the air at levels between 1.4 and 7.6 percent. A concentration of gasoline vapour in air below 1.4 percent is too "lean" to burn. Gasoline vapour levels above 7.6 percent are too "rich" to burn. Flammable limits, like flashpoints however, are intended as guides not as fine lines between safe and unsafe.
What is an Autoignition Temperature?
A material's autoignition or ignition temperature is the temperature at which a material self-ignites without any obvious sources of ignition, such as a spark or flame.
Most common flammable and combustible liquids have autoignition temperatures in the range of 300°C (572°F) to 550°C (1022°F). Some have very low autoignition temperatures. For example, ethyl ether has an autoignition temperature of 160°C (356°F) and its vapours have been ignited by hot steam pipes. Serious accidents have resulted when solvent-evaporating ovens were heated to temperatures above the autoignition temperature of the solvents used. Autoignition temperatures, however, are intended as guides, not as fine lines between safe and unsafe. Use all precautions necessary.
How can flammable and combustible liquids be a fire or explosion hazard?
At normal room temperatures, flammable liquids can give off enough vapour to form burnable mixtures with air. As a result, they can be a serious fire hazard. Flammable liquid fires burn very fast. They also give off a lot of heat and often clouds of thick, black, toxic smoke.
Combustible liquids at temperatures above their flashpoint also release enough vapour to form burnable mixtures with air. Hot combustible liquids can be as serious a fire hazard as flammable liquids.
Spray mists of flammable and combustible liquids in air may burn at any temperature if an ignition source is present. The vapours of flammable and combustible liquids are usually invisible. They can be hard to detect unless special instruments are used.
Most flammable and combustible liquids flow easily. A small spill can cover a large area of workbench or floor. Burning liquids can flow under doors, down stairs and even into neighbouring buildings, spreading fire widely. Materials like wood, cardboard and cloth can easily absorb flammable and combustible liquids. Even after a spill has been cleaned up, a dangerous amount of liquid could still remain in surrounding materials or clothing, giving off hazardous vapours.
What is the danger of flashback?
Vapours can flow from open liquid containers. The vapours from nearly all flammable and combustible liquids are heavier than air. If ventilation is inadequate, these vapours can settle and collect in low areas like sumps, sewers, pits, trenches and basements. The vapour trail can spread far from the liquid. If this vapour trail contacts an ignition source, the fire produced can flash back (or travel back) to the liquid. Flashback and fire can happen even if the liquid giving off the vapour and the ignition source are hundreds of feet or several floors apart.
Can flammable or combustible liquids be hazardous to my body?
The most obvious harm would be the danger of a fire or explosion. After the immediate danger of a fire, there are sometimes other properties of these liquids that may be hazardous to the body. Flammable and combustible liquids can also cause health problems depending on the specific material and route of exposure (breathing the vapour/mist, eye or skin contact, or swallowing). Some flammable and combustible liquids are corrosive. Many undergo dangerous chemical reactions if they contact incompatible chemicals such as oxidizing materials, or if they are stored improperly.
The Material Safety Data Sheet and the supplier's labels on the containers should tell you about all the hazards for the flammable and combustible liquids that you work with.
An example is 2-propanol (also known as: dimethylcarbinol, isopropanol, or isopropyl alcohol). It is a colourless liquid with a sharp odour like rubbing alcohol or resembling that of a mixture of ethanol and acetone. It is flammable liquid and vapour. Vapour is heavier than air and may spread long distances. Distant ignition and flashback are possible. It is also considered to be a mild central nervous system depressant. High vapour may cause headache, nausea, dizziness, drowsiness, incoordination, and confusion. It may also be irritating to the respiratory tract or eyes.
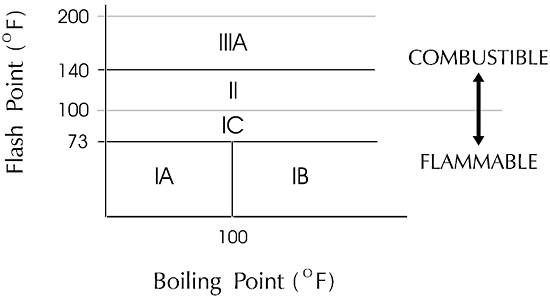
Combustible liquid: any liquid having a flash point at or above 100ºF (37.8ºC).
Combustible liquids shall be divided into two classes as follows:
-
1.Class II liquids shall include those with flash points at or above 100ºF (37.8ºC) and below 140ºF (60ºC), except any mixture having components with flash points of 200ºF (93.3ºC) or higher, the volume of which make up 99 percent or more of the total volume of the mixture.
-
2.Class III liquids shall include those with flash points at or above 140ºF (60ºC). Class III liquids are subdivided into two subclasses:
-
■Class IIIA liquids shall include those with flash points at or above 140ºF (60ºC) and below 200ºF (93.3ºC), except any mixture having components with flash points of 200ºF (93.3ºC), or higher, the total volume of which make up 99 percent or more of the total volume of the mixture.
-
■Class IIIB liquids shall include those with flash points at or above 200ºF (93.3ºC). This section does not regulate Class IIIB liquids. Where the term "Class III liquids" is used in this section, it shall mean only Class IIIA liquids.
-
When a combustible liquid is heated to within 30ºF (16.7ºC) of its flash point, it shall be handled in accordance with the requirements for the next lower class of liquids.
Flammable liquid: any liquid having a flash point below 100ºF (37.8ºC), except any mixture having components with flashpoints of 100ºF (37.8ºC) or higher, the total of which make up 99 percent or more of the total volume of the mixture. Flammable liquids shall be known as Class I liquids. Class I liquids are divided into three classes as follows:
-
1.Class IA shall include liquids having flash points below 73ºF (22.8ºC) and having a boiling point below 100ºF (37.8ºC).
-
2.Class IB shall include liquids having flash points below 73ºF (22.8ºC) and having a boiling point at or above 100ºF (37.8ºC).
-
3.Class IC shall include liquids having flash points at or above 73ºF (22.8ºC) and below 100ºF (37.8ºC).
It should be mentioned that flash point was selected as the basis for classification of flammable and combustible liquids because it is directly related to a liquid's ability to generate vapor, i.e., its volatility. Since it is the vapor of the liquid, not the liquid itself that burns, vapor generation becomes the primary factor in determining the fire hazard. The expression "low flash - high hazard" applies. Liquids having flash points below ambient storage temperatures generally display a rapid rate of flame spread over the surface of the liquid, since it is not necessary for the heat of the fire to expend its energy in heating the liquid to generate more vapor.
The above definitions for classification of flammable and combustible liquids are quite complex. The diagram below should aid in their understanding.
Classes of Flammable and Combustible Liquids
as Defined by 29 CFR 1910.106
FLAMMABLE (EXPLOSIVE) LIMITS
When vapors of a flammable or combustible liquid are mixed with air in the proper proportions in the presence of a source of ignition, rapid combustion or an explosion can occur. The proper proportion is called the flammable range and is also often referred to as the explosive range. The flammable range includes all concentrations of flammable vapor or gas in air, in which a flash will occur or a flame will travel if the mixture is ignited. There is a minimum concentration of vapor or gas in air below which propagation of flame does not occur on contact with a source of ignition. There is also a maximum proportion of vapor in air above which propagation of flame does not occur. These boundary-line mixtures of vapor with air are known as the lower and upper flammable limits (LFL or UFL) respectively, and they are usually expressed in terms of percentage by volume of vapor in air. See figure below.

In popular jargon, a vapor/air mixture below the lower flammable limit is too "lean" to burn or explode, and a mixture above the upper flammable limit is too "rich" to burn or explode. The LFL is also known as the lower explosive limit (LEL). The UFL is also referred to as the upper explosive limit (UEL). No attempt is made to differentiate between the terms flammable and explosive as applied to the lower and upper limits of flammability.
Back to Main Wax Removal page
Back to Main Pysankarstvo page
Flammable and Combustible Liquids